CTT’s radiotherapeutic product, CTT1403, is a PSMA-targeted 177Lu-labeled agent for prostate cancer that has excellent binding characteristics, long circulation half-life, enhanced tumor uptake and substantial anti-tumor efficacy. CTT1403 specifically delivers the radionuclide, 177Lu, to cancer cells, minimizing toxic side effects and more effectively treating metastatic prostate cancer and other tumors where PSMA is expressed. Recognizing the same PSMA target and utilizing the same binding region, CTT1403 and its companion agent, CTT1057, can be used as a theranostic pair to effectively detect and treat PSMA positive tumors.
Two features make CTT1403 unique and unlike other PSMA-targeted drugs currently in development: 1. CTT’s molecules bind irreversibly to PSMA and this distinctive mode of binding enhances rapid uptake of the drug within the tumor; and 2. CTT1403 contains an albumin binding component that increases circulation of the drug in the body and further increases the dose of drug that accumulates at the tumor sites.
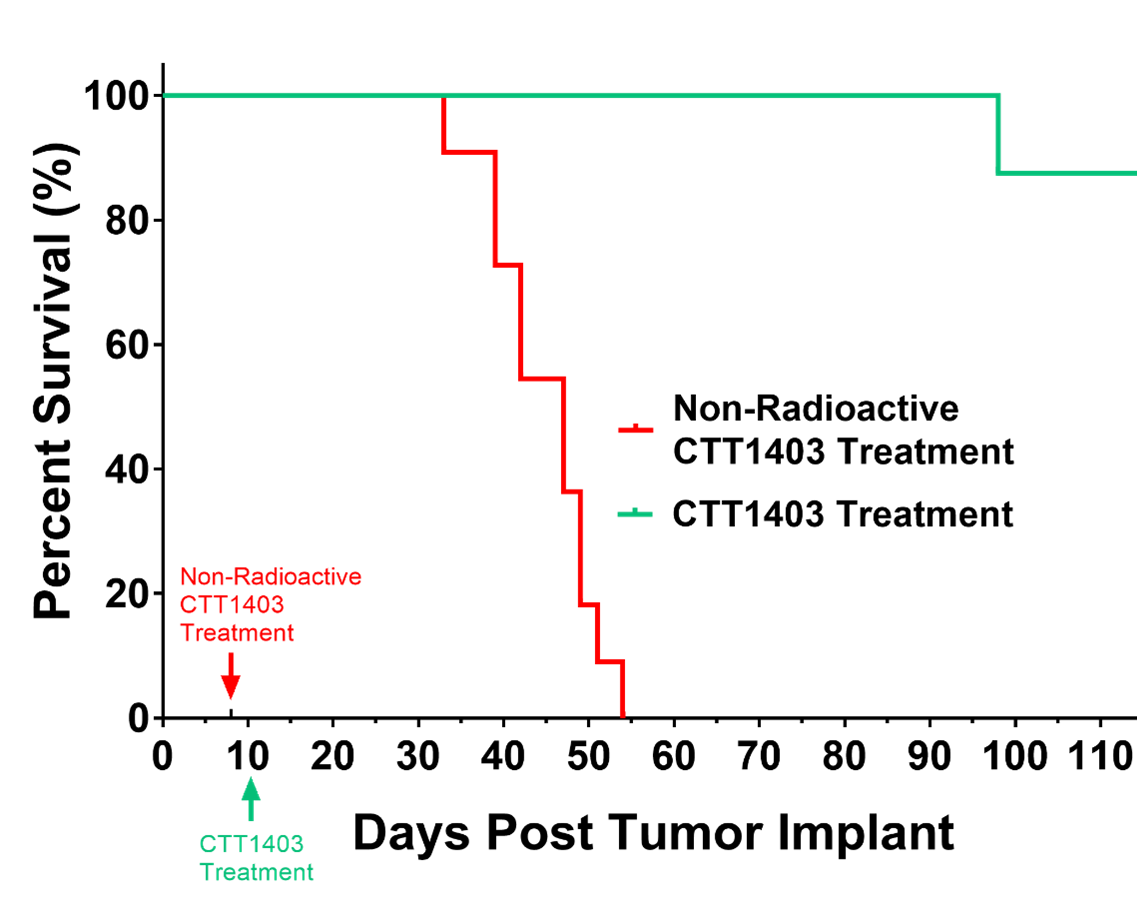
CTT1403 has strong pre-clinical data and has completed a Phase I clinical trial: ClinicalTrials.gov ID NCT03822871 https://clinicaltrials.gov/study/NCT03822871?term=CTT1403&rank=1 The preclinical data showed increased survival and reduction in tumor growth in animal models growing prostate tumors that express PSMA as shown in the survival curve. The Phase I clinical trial indicated that CTT1403 targeted PSMA expressing tumors and that the compound did not have any safety concerns.
Distinguishing Features of CTT1403
- Radiotherapy agent for prostate cancer with superior binding characteristics, long circulation half-life, enhanced tumor uptake and anti-tumor efficacy
- Recognizes a validated biomarker, PSMA, in a cancer with an unmet need
- Possibility for expansion into other cancers based on PSMA expression in the neovasculature
- Strong patent protection
Continuing development of CTT1403
CTT has completed the Phase I clinical trial of CTT1403 and has developed a formulation that is amenable to automated synthesis for further clinical use. The drug is available for licensing.